To Live or Not to Live
Dialysis is, aside from transplantation, the only treatment to kidney failure. Without this, the patient will die.
Chapter One of the book ‘‘Father of Artificial Organs’’ (life and work of the Dutch Doctor and Engineer Willem Kolff) reads
It is October 1938 when Dr Kolff, a young resident at the university hospital in Groningen (Netherlands), comes across a seriously ill young man in one of the four beds to which he has been assigned. The patient’s name is Jan Bruning, a twenty-two year old farmer’s son from Groningen. He is suffering from chronic inflammation of the kidneys, he has very high blood pressure and high pulse rate. He has not produced any urine for days and so the toxins in hics blood are no longer being excreted. His entire body hs swollen as a result. He is vomitting more and more often, his breath smells of urine. The end stage of uremia which entails a total failure of the kidney funcion, will cause his body to suffocate in his own waste products. The young man has insufferable itching over his entire body due to the crystallized deposits of urea that literally seeks a way out from his blood through the pores of the skin. He had become permanently blind the day before: his eyes were irreparably damaged due to the increasing pressure of crystallized urea behind his eye sockets. The headache must be excruciating but Jan Bruning no longer feels it. Following a long period of increasing mental confusion, convulsions, delirium and psychoses, he is slipping further and further in to a deep coma. The farmer’s son is dying.
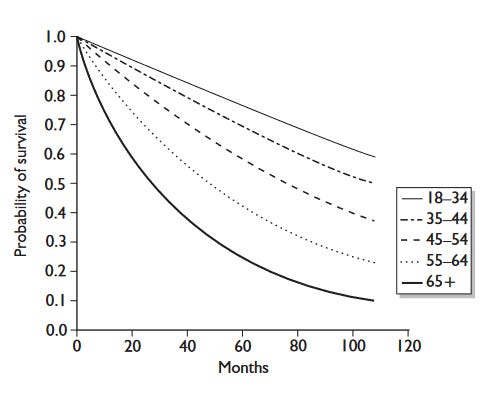
Dialysis is life-saving, yet still, patients on dialysis have much higher mortality risk compared to the general population. As you can see in the graph below, someone my age (29) on dialysis can expect to live another 18 years (to a ripe age of 47!). The survival curves are even more stark, with about 40% of my age group dying in 10 years of dialysis. With a transplant, the life expectancy goes up considerably to almost 40 years (I may see my grand-children (fingers crossed!)). This is however very much affected by the fact that transplants often fail within 15-20 years and that between transplant waiting can be considerably long.
The life expectancy on dialysis is profoundly low, but so is the quality of life. At the thought of returning to dialysis I have often fantasised upon, instead, ending up at the bottom of Al Legne.
Indeed, while on dialysis I wrote
There is a thread of narrative that is constantly running through my mind. Its a little devil that says, just stop the dialysis. There is a psychological burden associated with being dependent on a machine and a whole logistics network, to have the mark of disease follow you at all times. The little voice says, just stop, pass away and perhaps you'll roll again and be more fortunate. Of course I don't know whether I will roll again, or that I will be more fortunate, there are plenty a worse lives. Regardless, I don't heed this voice right now, I still feel like I have much to explore and to do. I pray the voice never becomes too commanding.
The reduction in quality of life is both psychological and physiological. Psychologically, there is the ‘‘mark of disease’’ I talk about above. There is the potent body dysmorphia you experience from having items literally alien to your body become a part of it. The dependency you feel on others, but also the anxiety from your inability to fulfil your social role. In terms of physical, there is the fatigue, nausea, headaches, the heavy fluid restrictions and the dietary restrictions.
I will go into each of these in much greater depth in follow-up posts, but suffice it to say, Al Legne becomes a potent attractor secondarily to its world-class climbing.
How To End Dialysis
Further incremental improvements to dialysis is not an option. We are well past the point of diminishing returns.
Therefore, any effort spent on promoting further improvements to dialysis are best spent in creating the paradigm shift to take us in to another exponential growth trajectory. ‘‘Lab grown organs’’ is one way to trigger this huge paradigm shift, not only for kidney disease but for everything. Indeed, a lot of international effort and funding is being spent advancing along this line for all sorts of healthcare applications. However, I don’t see this panning out in the medium term (<30 years).
In a lot of my work, I talk about orders of magnitude. This is a good way of assessing whether we can get something to do what we want. If getting cells to form vascularised (with blood flow) hierarchical structures, outside the embryological niche, in the ‘‘‘test-tube’’ via a process of morphogenesis, is two orders of magnitude harder than getting to organoids, we are out of luck. The fact that we can sustain adult organs outside the body in an ex-vivo perfusion machine for only 1 week for the liver, and 24 hours for the kidney affirms my suspicion that this problem, is hard. It is likely that we need to engage in advanced forms of fabrication and bioengineering to solve it.
The medium term solution will alleviate the suffering of those suffering now and in the near future. Those who will likely die before a long-term solution becomes available.
To solve dialysis in the medium term we need another solution. Some may say, why are we aiming for a medium term solution? The medium term solution will alleviate the suffering of those suffering now and in the near future. Those who will likely die before a long-term solution becomes available. Adittionally dialysis costs $35 Billion in the US with similar costs for the EU. With a medium term solution vast financial resources could be freed up to pursue long-term solutions.
In my opinion, There are two feasible ways of ending dialysis in the medium term. The first is xenotransplants where we have genetically engineered pigs whose tissue is compatible with our immune system. The other option is the Artificial Kidney.
The Artificial Kidney
In the literature, you often hear terms like ‘‘implantable artificial kidney’’ or ‘‘implantable bio-artificial kidney’’. These additions to the simple word ‘‘artificial kidney’’ is mainly because the dialysis machines have historically been referred to as ‘‘artificial kidneys’’. This is another misnomer. The dialysis machine is just that, a machine that dialyses the blood. Not only can it not recapitulate most of the physiological functions of the kidney, it is completely external to the body. So from hence, when I write ‘‘the artificial kidney’’ I mean a device, that is completely internal to the body, that may or may not have a cellular component but can regardless perform all the physiological functions of the kidney.
The artificial kidney is completely internal to the body, no external hoses, pipes needles or catheters. You are a self-contained human being, you are made whole.
These functions are:
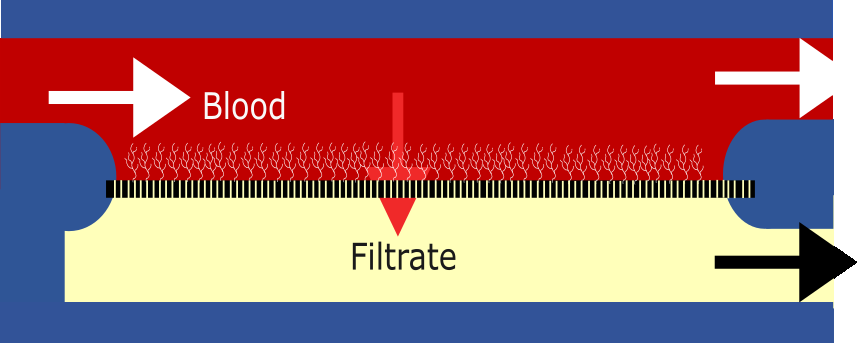
Blood filtration: The blood flows through the glomeruli and the pressure forces about 20% of the water content of the blood, ions (such as sodium, potassium), small useful molecules such as glucose and waste molecules such as urea and creatine, through the nano-pores of the slit diaphragm. This is called the filtrate, it’s not urine yet! The proteins which our body needs are bigger than the pores, so these are retained in the blood. This is the first stage
Resorption: About 200 L of filtrate is generated every day. If this would all pass to the bladder, we would quickly die from dehydration. So the epithelial cells of the kidney’s tubules must do the gargantuan task of resorbing back in to the blood stream 98% of this water. Moreover, the ion balance in the body must also be kept. So most of the sodium must also be taken back up. The leftover liquid is passed in to the bladder as urine.
Secretion: Some large waste molecules, namely those bound to the protein albumin (which is fortunately too large to pass the initial filter), need to be filtered out also. This is done in the tubules by active secretion of the tubular cells. Dialysis machines cannot do this active secretion. This causes a build-up of protein bound uremic toxins (PBUTs). For instance, β2 microglobulin can aggregate into amyloid fibres that deposit in joint spaces, a disease, known as dialysis-related amyloidosis.
To earn its name, the artificial kidney must recreate all these functions.
The Artificial Kidney Program
When it comes to the artificial kidney, here is what we envision it to look like. You have a kidney sized device, implanted in a similar region as a normal kidney transplant. It is composed of two parts, the hemofilter (analogous to the glomeruli) and the resorption unit (analogous to the tubule). The hemofilter has a blood inlet and outlet and the resorption/ion-exchange unit is connected to the bladder for the urine output.
Importantly, the artificial kidney is completely internal to the body, no external hoses, pipes needles or catheters. You are a self-contained human being, you are made whole.
The Hemofilter
High flux, ultra-thin, porous membrane. The membrane has <20nm pores that allow small solutes such as urea to pass through in to the ‘‘pre-urine’’ or the filtrate side. It is then coated with blood-compatible polymer such that there is no clotting of the blood or otherwise fouling of the membrane. There are different materials and structures we can use and are using to create this. Each have pros and cons but I won’t go into them just yet.
The Resorption/Ion-Exchange Unit
Currently, the only attempts at making this work is by integrating kidney cells in to the device. Indeed, our collaborators at the University of Utrecht are trying to do just this. The idea is to use a membrane with larger pores (~200nm) on the other side of which will be proximal tubule cells (cells closest to the glomerulus shown in the figure with the kidney). The cells form a monolayer, and they resorb the water and carry out electrolyte regulation with the blood-side. On the blood-side, there is again a blood-compatible polymer. At the end of the resorption process, there is urine
This idea has a lot of challenges too, namely keeping cells alive and functioning in an artificial construct. It is therefore our desire to also pursue a completely artificial method of doing the resorption, without cells. Here, we are in virgin scientific ground with a lot of uncertainty. But I feel like a thorough feasibility analysis of both approaches prior to committing too strongly to either is well advised.
The Timeline
I will not go into a lot of detail how exactly we will recreate these functions in this post. Instead, this will follow on in later posts, as I want to keep things currently fairly high-level. Here is a squiggly timeline of the program
The timeline above is not to my wife’s aesthetic taste, and it can be better. But then again, I am a grease monkey and getting me to make something visually appealing would (in-time) cost more than hiring a professional graphic designer, so we have this to work with. All in all, I want to have a working prototype by 2030. It must be able to keep a large animal with kidney failure alive until the device fails.
In the two components, hemo-filter and bio-reactor, it is essential to show that the device has all the properties we want it to have and that we can test on the bench top before animal experiments. This means the device has the required filtration rate to keep the animal alive (demonstrated on the bench-top) and that there is no other fault (i.e. braking of the filtration barrier, fluid leakage, lack of resorption etc). Only after this bench-top testing is done, would we move on to the animal testing.
Wrap Up
This is the Artificial Kidney Program with its motive and its plan.
I know it’s a very high-level overview of a lot of things. It actually feels kind of wrong to me to leave as it is, without reams of citations and arguments to back up my statements. Short of writing a couple of papers, I cannot go into each in the detail that I wish to. I will, however, in following posts, cover each topic as concisely as I allow myself to.
The next post will be about the Kidney Team pushing The Artificial Kidney Program forward!











Wow this mind blowing!!!!!