This post will be of a more technical nature. I want to elaborate a bit about the challenges facing the artificial kidney without sugar coating any of it, because they are substantial. Indeed, the blog would not be named as it is, if these challenges were not.
I will split this in to three posts; the blood filter, the resorption unit and the implantation.
The Blood Filter
The blood filter is the first step in the biological kidney, but also in the artificial kidney. Its role is to ensure proteins at the size of, or larger than albumin stay in the body. Albumin is by far the most dominant protein, usually blood contains 40 grams per liter, think egg-white, imagine filtering that. So our filtering membrane (sieve) needs to be small enough to reject albumin. Albumin is 8 nm in diameter and 15 nm long molecule. So with circular pores, 7nm diameter should be enough to reject all of albumin. But due to its irregular shape pores up to 13nm or so, may even be sufficient. These pores are very small indeed. On the highest end Scanning Electron Microscope we can just about make them out.
This very small pore size leads to the first great challenge, permeability.
Permeability
Take a membrane with pores as shown below (ignore the bushes for now). We will put blood in what is known as ‘cross-flow’. This means the flow of blood goes parallel to the surface of the membrane, rather than straight on, perpendicular to it. The perpendicular filtration is called ‘dead-end’. The grating that stops your food stuffs from going down the drain in your kitchen sink is operating under dead-end flow and it gets clogged fairly often. In cross-flow, the velocity of the fluid and the force that it imparts on the surface of the membrane, removes this surface fouling, much like a pressure washer.
If GFR drops below around 5, life becomes impossible and death follows. Permeability of the artificial kidneys’ blood filter needs to be at-least 15ml/min
The hydrostatic pressure, which in our case is blood pressure, forces some of this fluid and molecules smaller than the pore diameter, down through the membrane. This creates the filtrate.
Each Kidney can force a maximum of 60 ml/min of filtrate down the glomerular filter. For two kidneys this is 120 ml/min. This is known as the glomerular filtration rate (GFR). If GFR drops below around 5, life becomes impossible and death follows. At a GFR less than 15 ml/min many physical symptoms such as nausea, headaches, fatigue and gout show up. Gout is inflammation of the toe join caused by deposition of uric acid which forms needle like crystals. Uric acid accumulates because the glomerular filtration rate is too low and it can’t escape the body. This is extremely painful, and makes walking very hard.
Therefore, the permeability of the artificial kidneys’ blood filter needs to be at-least 15ml/min, this is almost 1l/hour.
Now you may be thinking, that seems low enough to be feasible. But due to the very small pore size it is still a big challenge. The hydraulic permeability of a single pore has the following proportionality relationship:
This just means that the flow through the pore is proportional to the radius of the pore to the power of 4, divided by the length of the pore. The effect of pore size is very large. If I increase the radius by a factor of 2, the flow increases by a factor of 16. This makes intuitive sense. If you try to blow air through a very thin straw, its very difficult, you need a lot of pressure or the flow rate is low. If this staw is extra long, this becomes extra difficult. Our membranes need to have very small pores ~ 10nm so they must also be very very thin, ~10-100 nm to try to offset the small pore size to therefore have the required flow rate we need.
The challenge is synthesizing pores that are small enough at high enough porosity.
Conventional dialysis membranes are quiet thick and have very disperse pore sizes. Polyflux which is a state of the art hemodiafiltration unit has a clearance of 85ml/min for an area of 1.4 m2. This seems greater than 15 ml/min, but the area is huge! You can’t implant this in a person, there is no space. The total filtration area I am aiming for 0.04m2. For this area, state of the art dialysis membranes give 2.42 ml/min filtration, so, they won’t work. We need a factor of 10 over this yet.
If we make the membranes thinner, of course our permeability increases. For polymeric membranes controlling this thickness is feasible but not as easy as controlling thickness in inorganic membranes.
If we make our membranes thinner we make them weaker. This means that the membranes might not tolerate the pressures we apply. I can easily foresee maximum blood pressure being 200 mmHg, this can happen when someone with naturally high blood pressure stands up and initiates exercise. We therefore need membranes to easily tolerate 400-600 mmHg of pressure (so a safety factor of 2-3). Whilst also being fatigue resistant.
So there must be a balance between strength (hence thickness), and permeability. One important factor is the material itself. Take Silicon for instance. This is a very brittle material, but Silicon Nitride on the other hand is much stronger (up to 4x as strong). With 4x strong material permeability can be increased massively, because we can have a very thin membrane window (down to 50nm) that spans a fairly large space (up to millimeters). This is one of my prime criticisms of The Kidney Project. That for some inexplicable reason they have converged upon an inferior material and, in my opinion, making things harder than they should be. But this is a topic for another day.
In addition to the material itself, hierarchy can be employed to support a thin layer with very small pores, with a bottom thicker layer with larger pores/structures. Imagine the above membrane with many small windows, separated by thin strips of thicker, non-membrane material. Tuning these structures appropriately requires iterations, but I don’t think this is a very large challenge. The challenge is synthesizing pores that are small enough at high enough porosity.
[Trying to remove the SiN membrane from the gel-pack. I find this incredibly impressive. You see this 50nm thickness membrane buckle and distort but still not fracture. You can see the world in a greenish hue if you look through the membrane, which you can easily do, because it is so thin!]
With chemical and electro-chemical etching techniques the smallest pore-size that can be comfortably attained is around 15nm. This would still allow albumin to pass. We may be able to produce smaller pores, but continuous membrane with arrays of pores at the scale we require to be industrially viable is not feasible with these other techniques. Therefore, the pores must be shrunk further with surface coatings of either organic of inorganic nature. Organic surface coatings are of the type that people use for blood compatibility (more on this later). In-organic coatings include atomic deposition of very thin layer of mainly ceramics. Such as Aluminium Oxide. These would then shrink the pores, from the minimum we can make them (15-20nm), to the dimensions we need ~10nm. Of course during this shrinking process we lose a lot of permeability due to the strong effect of pore radius on the flow as per the equation above.
Because the blood filter must filter blood.
It is this pore-shrinking step that is challenging. It may be feasible to create an ultra-thin (~1-4nm) but stable cake-layer, with the size selectivity we want, on top of these ~15-20nm pores essentially bridging them. This would create the selectivity we want, whilst not reducing the permeability by much. I am currently studying coating/pore interaction with my colleagues Dr. Jeroen Vollenbroek (UMCU & TU Twente) and Dr. Bijoy Bera (TU Delft). I believe that the membrane problem is challenging, though looking outside the box of the paradigm set by The Kidney Project, we are at a good place to crack it.
Cracking the problem would mean we would only need 10 membranes of 4x10cm dimension stacked on top of each other with blood flow in a device, which in total is 0.04m2. But having this doesn’t mean we are through the woods as far as the blood filter is concerned.
Because the blood filter must filter blood.
Blood Compatibility
There are two problems here one is fouling, the other is coagulation and thrombus risk. Let us first look at fouling.
Fouling
Fouling occurs when we have proteins, cells and other substances adsorb or otherwise coat the membrane and its pores. This can greatly reduce the permeability of the membrane below the 15 ml/min cut-off. Indeed, given that our pores are so small, it doesn’t take much.

The figure above shows just how profound this can be. Here we have albumin fouling on an ultra-filtration membrane. Even though the albumin concentration is fairly low 0.3g/L, compared to the concentration in the body ~40g/L, the fouling effect is huge, almost a factor of 5 decrease in permeability over a period of 3 hours.
To reduce fouling we are currently using organic surface coatings, which also help shrink the pores. One such coating that is typically used is PEG.
The PEG can form hydration layers due to the hydrogen bonds with water, which act as energy barriers for protein adsorption. This is brilliant. However PEG coatings over long period of time are unstable. Therefore we are exploring ways of increasing PEG stability.
Another way of reducing the fouling is by using charge. A large number of proteins, and albumin especially is negatively charged. If the surface charge of our membrane/coating is also negative, proteins will be repelled from the surface and so fouling will not occur. At-least this is the idea. This also means that we can get away with larger pores and still reject albumin due to electrostatic repulsion. I am currently exploring this at TU Delft will have some preliminary data soon.
However negative charged surfaces can trigger the coagulation cascade. Is there really no such thing as a free lunch?
Coagulation
Factor XII, also known as the Hageman factor has a net positive charge and interacts with negatively charged surfaces to trigger the intrinsic pathway of coagulation and so thrombus. Therefore, our surfaces cannot have a strong negative charge. However, adsorption of for instance, fibrinogen (see figure below) which is negatively charged, can also lead to coagulation. So in this case negative charging would not only stop general protein fouling and loss of permeability but also possibly coagulation via fibrinogen adsorption also. The trick I imagine is to balance the charge density.
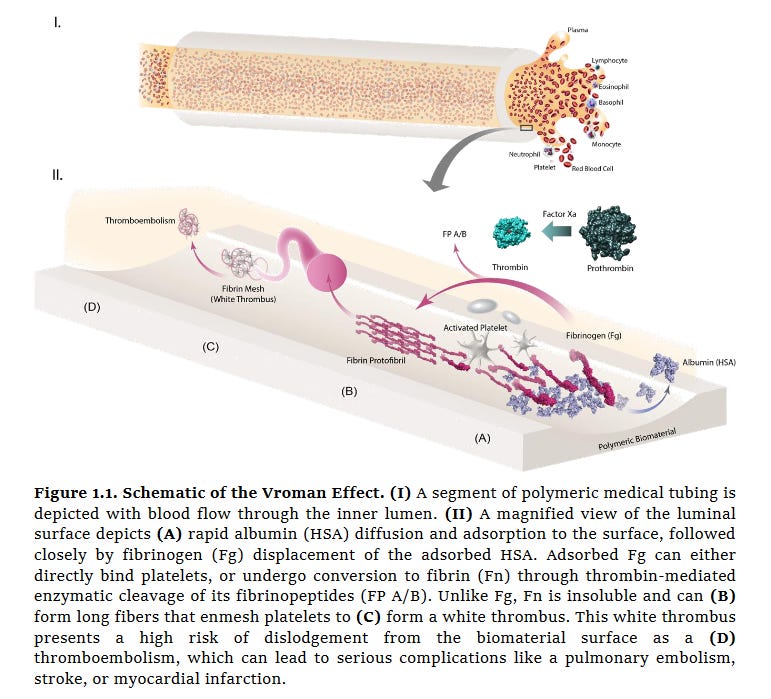
Of course, one can go for an uncharged, PEG based approach. In this case the trick would be to ensure PEG stays put over the operational lifetime of the Artificial Kidney.
The danger with any coagulation is the creation of a thrombus which could be fatal. But also the general reduction in device performance below the 15ml/min cutoff.
The reason for why biomaterials have it so rough in the body is that they lack the antithrombotic properties of the endothelium, i.e. the cells which line our blood vessels. However, it can be that with the right coating we can promote endothellilisation of the Artificial Kidney. I view this as a longer term goal. With a healthy endothelial layer the Artificial Kidney would remain functioning for much longer than with an artificial surface coating. Of course, we must make sure that this new endothelium does not reduce our permeability by forming a tight layer over the membranes. Or otherwise, the surface coating we applied to facilitate the inntial blood compatibility and endothelilisation must be biodegradable and its replacement by the endothelium wouldn’t reduce the permeability. Again, I see this as a longer term concern, regardless I aim to start experiments on this as soon as possible to help identify future problems, or opportunities!
I believe that we will also need active anti-fouling and active anti-thrombotic mechanisms in the Artificial Kidney. I will start looking in to this in tandem with blood-compatibility of our surfaces very soon.
Summary
Overall, there are two great challanges. Permeability and blood compatibility. These two challanges are interracting, with blood compatibility being the governing factor behind also the permeability. I think we can make membranes that are permeable with the right pore sizes. However, having the right surface coating for blood-compatibility such that the surface triggers neither Factor XII nor fibrinogen and remains in general, low fouling is tricky. The right surface coating here will also determine how our pores shrink and therefore the permeability. There is some iterative work to be done between the two parts.
But I believe we can overcome these problems in the next two years. I always take the example of the Total Artificial heart. What was once a dream, is now a part of reality. All thanks to the concerted effort of a lot of people.









Great job! You are aware of all hurdles and looks like you re overcoming them in the end!